
Editor’s note: Hear directly from pancreatic cancer researcher Kirsten Bryant, PhD, and Julie Fleshman, president and CEO of the Pancreatic Cancer Action Network, in this CBSN interview conducted on March 4, 2019.
An unexpected laboratory finding points to a new combination treatment strategy for pancreatic cancer, report two studies published in Nature Medicine today.
The papers – one study primarily out of the Lineberger Comprehensive Cancer Center at the University of North Carolina, and the other from Huntsman Cancer Institute at the University of Utah – look at using drugs that target proteins that are activated by KRAS in combination with a drug that blocks a process called autophagy.
KRAS, the most commonly mutated protein in pancreatic tumors, has notoriously been dubbed “undruggable,” because previous efforts to therapeutically block it directly have been unsuccessful. So, the investigators looked to turn off what gets turned on by mutant KRAS – as a surrogate for stopping KRAS activity itself.
But, the researchers quickly found that blocking proteins that get activated by KRAS is not sufficient to stop pancreatic cancer cell growth.

The lab groups of Channing Der, PhD (far left), and close collaborator and study coauthor Adrienne Cox, PhD (far right).
Kirsten Bryant, PhD, first author of the paper from the Lineberger Cancer Center group, joined the lab of renowned KRAS expert Channing Der, PhD, as a postdoctoral fellow in 2013. Bryant and Der are both recipients of Pancreatic Cancer Action Network (PanCAN) research grants, which contributed to the newly published study.
Bryant’s primary interest was pancreatic cancer metabolism, or the process by which cancer cells gather and utilize nutrients differently than healthy cells. Although initially skeptical of the relationship between KRAS and metabolism, Der gave Bryant the opportunity to pursue her scientific interests.
“Autophagy is one of the unusual ways that pancreatic cancer cells undergo metabolism; the cells basically begin eating themselves,” Bryant explained. “This self-eating allows cells to gain important nutrients from their own internal components.”
The link between protein signaling generated by mutant KRAS and autophagy was not well understood. However, Bryant recalls, she assumed that blocking KRAS would also stop cells from engaging in autophagy – since many processes that take place within pancreatic cancer cells are directly or indirectly orchestrated by mutant KRAS.
“We were surprised to find the opposite was true – autophagy got even stronger when KRAS signaling was turned off,” she said.
Another surprise was in store for Bryant and Der.
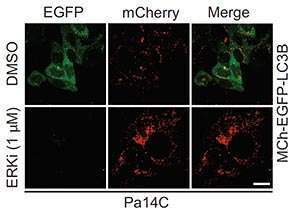
Unexpectedly, blocking signaling from mutant KRAS in pancreatic cancer cells led to an increase in autophagy, as shown by the increase in red staining and decrease in green in the ERKi row.
“I presented my results about inhibiting ERK (one of the proteins that gets activated by KRAS) and seeing an increase in autophagy at a conference in December 2017,” Bryant said. She recalls a fellow scientist, Martin McMahon, PhD, showing great interest in her poster presentation.
A few days later, McMahon emailed Der and explained that his research team had made similar discoveries – except they’d focused on a protein called MEK within the KRAS signaling pathway, instead of ERK.
“Rather than race to see who publishes the story first, we decided to meet regularly and then co-submit our manuscripts,” Bryant said.
“The papers are stronger together.”
In addition to a deep sense of validation upon seeing an independent research team replicate similar findings to theirs, Bryant was astonished to learn that Conan Kinsey, MD, PhD, postdoctoral fellow in McMahon’s lab and a medical oncologist, had treated a patient with the proposed combination therapy.
Although he’s since died, the patient experienced more than five months of progression-free survival after seemingly exhausting other treatment options available to him.

Bryant holds a photo of her father in front of the Capitol building at PanCAN’s National Pancreatic Cancer Advocacy Day.
This is a deeply personal fight for Bryant, who lost her father to pancreatic cancer in 2013 – only 11 months after his diagnosis.
“When I think about the patient Conan treated, I’m reminded of my dad,” Bryant said. “He, too, had run out of options, and while my research can no longer help him, I’m filled with guarded pride to think the work I’m doing could help others.”
Bryant added, “I put my dad’s name in the acknowledgements section of the paper, typically reserved for funding sources and individuals who’ve supported the science or the preparation of the paper.
“He’s thanked for ‘continued inspiration.’”
As for next steps, Bryant said she’s pursuing other ways to block signaling from KRAS and working to find more effective strategies to inhibit autophagy. And, both Der and McMahon’s groups are setting up clinical trials to formally test the combination treatment approach and determine its safety and effectiveness in pancreatic cancer patients.
“I’m so proud that I’m doing what I set out to do,” Bryant said, “and none of this would have been possible without PanCAN.”





